To avoid the cross contamination, the endoscope ought to be disinfected after using. But how to accurately evaluate the disinfection efficacy, it has been a difficult problem for a long time.
TAILIN innovatively developed the endoscope microbial limit test system to solve this problem.
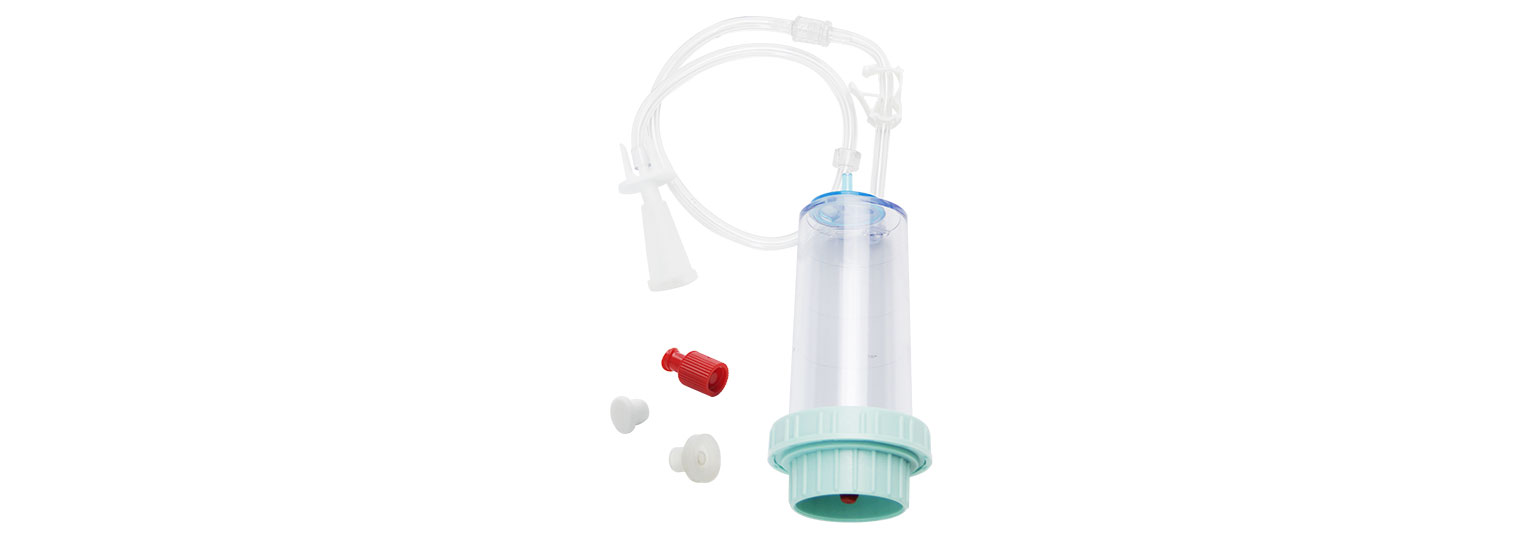
The Endoscope Microbial Test System consists of HTY-WP02 peristaltic pump for sampling,HTY-101 diaphragm pump for suction filtering, and FC501 filtration cup.
Step 1: Rinse the endoscope with sterile water, and transferring the rinsed water in FC501 filtration cup by HTY-WP02 peristaltic pump;
Step 2: Install the FC501 on the HTY-101 diaphragm pump;
Step 3: Start the HTY-101 diaphragm pump, the sampling rinsed water will filtering through theFC501 filtration cup.
Step 4: The microorganism will be hold on the filtration membrane, if there is any.
Step 5: Put the filtration membrane in the culture media for incubation and counting.
The Endoscope Microbial System can fit for most of endoscope brands; it is closed system which can avoid the external contamination when checking the endoscope disinfection/cleaning efficacy.
Luer threading connector for tight sealing and easy to dismantle;
Luer plug to avoid liquid drip;
Special designed connection to syringe;
High rigid and transparent cup body;
High flow rate filtration membrane to guarantee the microbial recovery.
Cup volume: 120 mL
Filtration membrane: MCE
Membrane pore size: 0.45μm
Ethylene Oxide (EO)sterilization
Consumables used for the sampling during endoscope microbial test.
Technical specifications are subject to change without notice. Copyright reserved by Tailin.


 CN
CN